
MPBH
(Protein Crosslinker heterobifunctional)
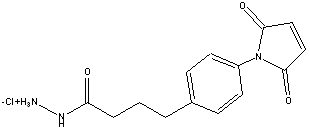
CL216
4-(N-Maleimidophenyl)butyric acid hydrazide hydrochloride
Formula Weight: f.w.: 309.7 daltons
MPBH is a long, maleimide-and-hydrazide crosslinker for conjugating sulfhydryls (cysteines) to carbonyls (aldehyde or ketones, such as those formed by oxidation of glycoprotein carbohydrates).
Pricing & Ordering
| Quantity | Price | Action |
|---|---|---|
| 50mgm + | $284.00 | |
| 100mgm + | $536.00 |
Spacer arm 17.9 angstroms. Heterobifunctional crosslinker. Carbohydrate selective and sulfhydyl reactive. MPBH has an oxidized carbohydrate specific hydrazide, a sulfhydryl reactive group and a spacer arm to accommodate a wide range of molecular coupling manipulations.
1.Chamow, S.M., Kogan, T.P., Peers, D.H., Hastings, R.C., Byrn, R.A. and Askenaszi, A. (1992). Conjugation of soluble CD4 without loss of biological activity via a novel carbohydrate-directed crosslinking reagent. J. Biol. Chem. 267(22), 15916-15922.
• Has an oxidized carbohydrate specific hydrazide, a sulfhydryl reactive group and spacer arms to accommodate a wide range of molecular coupling demands • -SH specific group is a maleimide that yields a thioether linkage upon coupling • Stable in DMSO • Reactive groups: hydrazide and maleimide • Reactive towards: carbohydrate and sulfhydryl groups • Use with EDC for reactivity toward carboxyl groups Product Details:

